Cells manage a wide range of functions in their tiny package — growing, moving, housekeeping, and so on — and most of those functions require energy. But how do cells get this energy in the first place? And how do they use it in the most efficient manner possible?
Where Do Cells Obtain Their Energy?

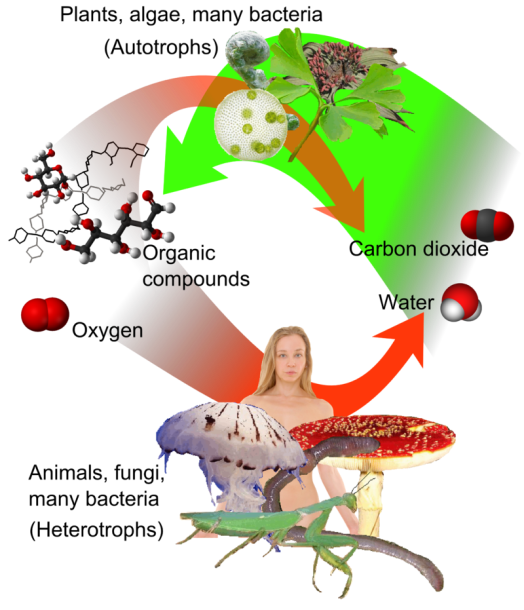
Cells, like humans, cannot generate energy without locating a source in their environment. However, whereas humans search for substances like fossil fuels to power their homes and businesses, cells seek their energy in the form of food molecules or sunlight. In fact, the Sun is the ultimate source of energy for almost all cells, because photosynthetic prokaryotes, algae, and plant cells harness solar energy and use it to make the complex organic food molecules that other cells rely on for the energy required to sustain growth, metabolism, and reproduction (Figure 1).
Cellular nutrients come in many forms, including sugars and fats. In order to provide a cell with energy, these molecules have to pass across the cell membrane, which functions as a barrier — but not an impassable one. Like the exterior walls of a house, the plasma membrane is semi-permeable. In much the same way that doors and windows allow necessities to enter the house, various proteins that span the cell membrane permit specific molecules into the cell, although they may require some energy input to accomplish this task.
How Do Cells Turn Nutrients into Usable Energy?
Complex organic food molecules such as sugars, fats, and proteins are rich sources of energy for cells because much of the energy used to form these molecules is literally stored within the chemical bonds that hold them together. Scientists can measure the amount of energy stored in foods using a device called a bomb calorimeter. With this technique, food is placed inside the calorimeter and heated until it burns. The excess heat released by the reaction is directly proportional to the amount of energy contained in the food.
Photosynthesis
Photosynthesis is a process used by plants and other organisms to convert light energy into chemical energy that can later be released to fuel the organisms’ activities (energy transformation). This chemical energy is stored in carbohydrate molecules, such as sugars, which are synthesized from carbon dioxide and water – hence the name photosynthesis, from the Greek φῶς, phōs, “light”, and σύνθεσις, synthesis, “putting together”. In most cases, oxygen is also released as a waste product. Most plants, most algae, and cyanobacteria perform photosynthesis; such organisms are called photoautotrophs. Photosynthesis is largely responsible for producing and maintaining the oxygen content of the Earth’s atmosphere, and supplies all of the organic compounds and most of the energy necessary for life on Earth.
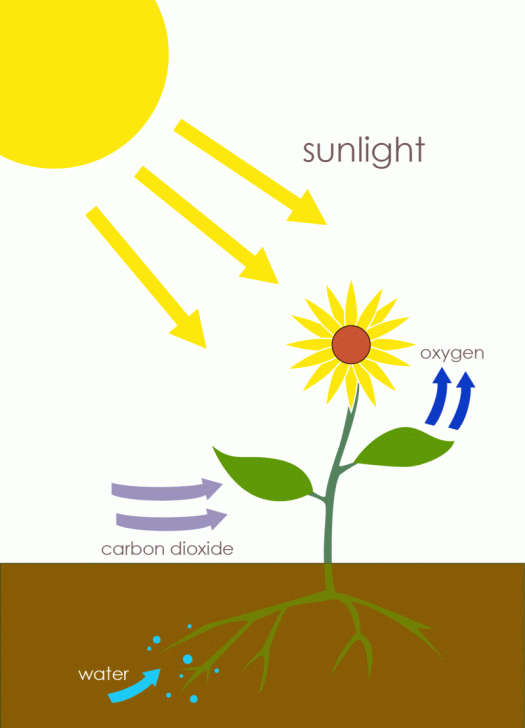
Although photosynthesis is performed differently by different species, the process always begins when energy from light is absorbed by proteins called reaction centres that contain green chlorophyll pigments. In plants, these proteins are held inside organelles called chloroplasts, which are most abundant in leaf cells, while in bacteria they are embedded in the plasma membrane. In these light-dependent reactions, some energy is used to strip electrons from suitable substances, such as water, producing oxygen gas. The hydrogen freed by the splitting of water is used in the creation of two further compounds that act as an immediate energy storage means: reduced nicotinamide adenine dinucleotide phosphate (NADPH) and adenosine triphosphate (ATP), the “energy currency” of cells.
In plants, algae and cyanobacteria, long-term energy storage in the form of sugars is produced by a subsequent sequence of light-independent reactions called the Calvin cycle; some bacteria use different mechanisms, such as the reverse Krebs cycle, to achieve the same end. In the Calvin cycle, atmospheric carbon dioxide is incorporated into already existing organic carbon compounds, such as ribulose bisphosphate (RuBP). Using the ATP and NADPH produced by the light-dependent reactions, the resulting compounds are then reduced and removed to form further carbohydrates, such as glucose.
The first photosynthetic organisms probably evolved early in the evolutionary history of life and most likely used reducing agents such as hydrogen or hydrogen sulfide, rather than water, as sources of electrons. Cyanobacteria appeared later; the excess oxygen they produced contributed directly to the oxygenation of the Earth, which rendered the evolution of complex life possible. Today, the average rate of energy capture by photosynthesis globally is approximately 130 terawatts, which is about three times the current power consumption of human civilization. Photosynthetic organisms also convert around 100–115 thousand million metric tonnes of carbon into biomass per year.
Photosynthesis Reactions
Light-dependent reactions
In the light-dependent reactions, one molecule of the pigment chlorophyll absorbs one photon and loses one electron. This electron is passed to a modified form of chlorophyll called pheophytin, which passes the electron to a quinone molecule, starting the flow of electrons down an electron transport chain that leads to the ultimate reduction of NADP to NADPH. In addition, this creates a proton gradient (energy gradient) across the chloroplast membrane, which is used by ATP synthase in the synthesis of ATP. The chlorophyll molecule ultimately regains the electron it lost when a water molecule is split in a process called photolysis, which releases a dioxygen (O2) molecule as a waste product.
The overall equation for the light-dependent reactions under the conditions of non-cyclic electron flow in green plants is:
- 2 H2O + 2 NADP+ + 3 ADP + 3 Pi + light → 2 NADPH + 2 H+ + 3 ATP + O2
Not all wavelengths of light can support photosynthesis. The photosynthetic action spectrum depends on the type of accessory pigmentspresent. For example, in green plants, the action spectrum resembles the absorption spectrum for chlorophylls and carotenoids with absorption peaks in violet-blue and red light. In red algae, the action spectrum is blue-green light, which allows these algae to use the blue end of the spectrum to grow in the deeper waters that filter out the longer wavelengths (red light) used by above ground green plants. The non-absorbed part of the light spectrum is what gives photosynthetic organisms their color (e.g., green plants, red algae, purple bacteria) and is the least effective for photosynthesis in the respective organisms.
Light-independent reactions
Calvin cycle
In the light-independent (or “dark”) reactions, the enzyme RuBisCO captures CO2 from the atmosphere and, in a process called the Calvin-Benson cycle, it uses the newly formed NADPH and releases three-carbon sugars, which are later combined to form sucrose and starch. The overall equation for the light-independent reactions in green plants is
- 3 CO2 + 9 ATP + 6 NADPH + 6 H+ → C3H6O3-phosphate + 9 ADP + 8 Pi + 6 NADP+ + 3 H2O
Carbon fixation produces the intermediate three-carbon sugar product, which is then converted to the final carbohydrate products. The simple carbon sugars produced by photosynthesis are then used in the forming of other organic compounds, such as the building material cellulose, the precursors for lipid and amino acid biosynthesis, or as a fuel in cellular respiration. The latter occurs not only in plants but also in animals when the energy from plants is passed through a food chain.
The fixation or reduction of carbon dioxide is a process in which carbon dioxide combines with a five-carbon sugar, ribulose 1,5-bisphosphate, to yield two molecules of a three-carbon compound, glycerate 3-phosphate, also known as 3-phosphoglycerate. Glycerate 3-phosphate, in the presence of ATP and NADPH produced during the light-dependent stages, is reduced to glyceraldehyde 3-phosphate. This product is also referred to as 3-phosphoglyceraldehyde (PGAL) or, more generically, as triose phosphate. Most (5 out of 6 molecules) of the glyceraldehyde 3-phosphate produced is used to regenerate ribulose 1,5-bisphosphate so the process can continue. The triose phosphates not thus “recycled” often condense to form hexosephosphates, which ultimately yield sucrose, starch and cellulose. The sugars produced during carbon metabolism yield carbon skeletons that can be used for other metabolic reactions like the production of amino acids and lipids.
Carbon concentrating mechanisms
On land
In hot and dry conditions, plants close their stomata to prevent water loss. Under these conditions, CO2 will decrease and oxygen gas, produced by the light reactions of photosynthesis, will increase, causing an increase of photorespiration by the oxygenase activity of ribulose-1,5-bisphosphate carboxylase/oxygenase and decrease in carbon fixation. Some plants have evolved mechanisms to increase the CO2 concentration in the leaves under these conditions.
Plants that use the C4 carbon fixation process chemically fix carbon dioxide in the cells of the mesophyll by adding it to the three-carbon molecule phosphoenolpyruvate (PEP), a reaction catalyzed by an enzyme called PEP carboxylase, creating the four-carbon organic acid oxaloacetic acid. Oxaloacetic acid or malate synthesized by this process is then translocated to specialized bundle sheath cells where the enzyme RuBisCO and other Calvin cycle enzymes are located, and where CO2 released by decarboxylation of the four-carbon acids is then fixed by RuBisCO activity to the three-carbon 3-phosphoglyceric acids. The physical separation of RuBisCO from the oxygen-generating light reactions reduces photorespiration and increases CO2 fixation and, thus, the photosynthetic capacity of the leaf. C4 plants can produce more sugar than C3 plants in conditions of high light and temperature. Many important crop plants are C4 plants, including maize, sorghum, sugarcane, and millet. Plants that do not use PEP-carboxylase in carbon fixation are called C3 plants because the primary carboxylation reaction, catalyzed by RuBisCO, produces the three-carbon 3-phosphoglyceric acids directly in the Calvin-Benson cycle. Over 90% of plants use C3 carbon fixation, compared to 3% that use C4 carbon fixation;however, the evolution of C4 in over 60 plant lineages makes it a striking example of convergent evolution.
Xerophytes, such as cacti and most succulents, also use PEP carboxylase to capture carbon dioxide in a process called Crassulacean acid metabolism (CAM). In contrast to C4 metabolism, which spatially separates the CO2 fixation to PEP from the Calvin cycle, CAM temporally separates these two processes. CAM plants have a different leaf anatomy from C3 plants, and fix the CO2 at night, when their stomata are open. CAM plants store the CO2 mostly in the form of malic acid via carboxylation of phosphoenolpyruvate to oxaloacetate, which is then reduced to malate. Decarboxylation of malate during the day releases CO2 inside the leaves, thus allowing carbon fixation to 3-phosphoglycerate by RuBisCO. Sixteen thousand species of plants use CAM.
In water
Cyanobacteria possess carboxysomes, which increase the concentration of CO2 around RuBisCO to increase the rate of photosynthesis. An enzyme, carbonic anhydrase, located within the carboxysome releases CO2 from the dissolved hydrocarbonate ions (HCO−
3). Before the CO2 diffuses out it is quickly sponged up by RuBisCO, which is concentrated within the carboxysomes. HCO−
3 ions are made from CO2 outside the cell by another carbonic anhydrase and are actively pumped into the cell by a membrane protein. They cannot cross the membrane as they are charged, and within the cytosol they turn back into CO2 very slowly without the help of carbonic anhydrase. This causes the HCO−
3 ions to accumulate within the cell from where they diffuse into the carboxysomes. Pyrenoids in algae and hornworts also act to concentrate CO2 around rubisco.
Cellular Respiration
Cellular respiration is a set of metabolic reactions and processes that take place in the cells of organisms to convert biochemical energy from nutrients into adenosine triphosphate (ATP), and then release waste products. The reactions involved in respiration are catabolic reactions, which break large molecules into smaller ones, releasing energy in the process, as weak so-called “high-energy” bonds are replaced by stronger bonds in the products. Respiration is one of the key ways a cell releases chemical energy to fuel cellular activity. Cellular respiration is considered an exothermic redox reaction which releases heat. The overall reaction occurs in a series of biochemical steps, most of which are redox reactions themselves. Although technically, cellular respiration is a combustion reaction, it clearly does not resemble one when it occurs in a living cell because of the slow release of energy from the series of reactions.
Nutrients that are commonly used by animal and plant cells in respiration include sugar, amino acids and fatty acids, and the most common oxidizing agent (electron acceptor) is molecular oxygen (O2). The chemical energy stored in ATP (its third phosphate group is weakly bonded to the rest of the molecule and is cheaply broken allowing stronger bonds to form, thereby transferring energy for use by the cell) can then be used to drive processes requiring energy, including biosynthesis, locomotion or transportation of molecules across cell membranes.
Aerobic respiration
Aerobic respiration requires oxygen (O2) in order to create ATP. Although carbohydrates, fats, and proteins are consumed as reactants, it is the preferred method of pyruvatebreakdown in glycolysis and requires that pyruvate enter the mitochondria in order to be fully oxidized by the Krebs cycle. The products of this process are carbon dioxide and water, but the energy transferred is used to break bonds in ADP as the third phosphate group is added to form ATP (adenosine triphosphate), by substrate-level phosphorylation, NADH and FADH2
| Simplified reaction: | C6H12O6 (s) + 6 O2 (g) → 6 CO2 (g) + 6 H2O (l) + heat |
| ΔG = −2880 kJ per mol of C6H12O6 |
The negative ΔG indicates that the reaction can occur spontaneously.
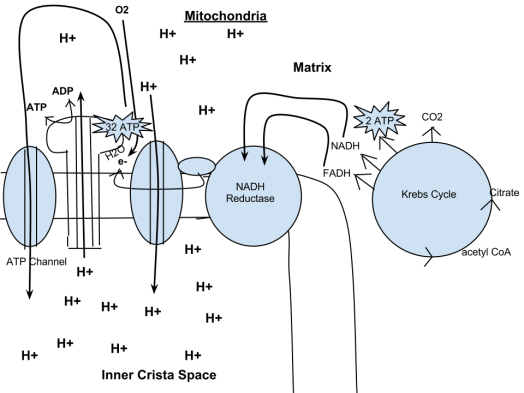
The potential of NADH and FADH2 is converted to more ATP through an electron transport chain with oxygen as the “terminal electron acceptor”. Most of the ATP produced by aerobic cellular respiration is made by oxidative phosphorylation. This works by the energy released in the consumption of pyruvate being used to create a chemiosmotic potentialby pumping protons across a membrane. This potential is then used to drive ATP synthase and produce ATP from ADP and a phosphate group. Biology textbooks often state that 38 ATP molecules can be made per oxidised glucose molecule during cellular respiration (2 from glycolysis, 2 from the Krebs cycle, and about 34 from the electron transport system). However, this maximum yield is never quite reached because of losses due to leaky membranes as well as the cost of moving pyruvate and ADP into the mitochondrial matrix, and current estimates range around 29 to 30 ATP per glucose.
Aerobic metabolism is up to 15 times more efficient than anaerobic metabolism (which yields 2 molecules ATP per 1 molecule glucose). However some anaerobic organisms, such as methanogens are able to continue with anaerobic respiration, yielding more ATP by using other inorganic molecules (not oxygen) as final electron acceptors in the electron transport chain. They share the initial pathway of glycolysis but aerobic metabolism continues with the Krebs cycle and oxidative phosphorylation. The post-glycolytic reactions take place in the mitochondria in eukaryotic cells, and in the cytoplasm in prokaryotic cells.
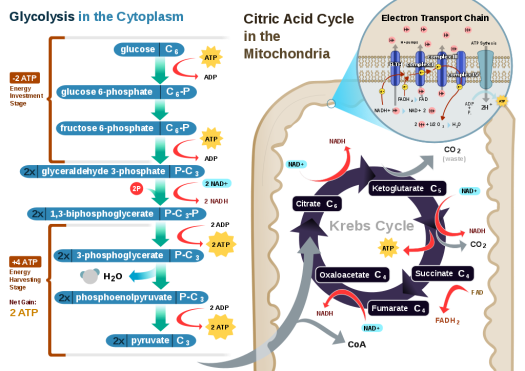
Glycolysis
Glycolysis is a metabolic pathway that takes place in the cytosol of cells in all living organisms. This pathway can function with or without the presence of oxygen. In humans, aerobic conditions produce pyruvate and anaerobic conditions produce lactate. In aerobic conditions, the process converts one molecule of glucose into two molecules of pyruvate(pyruvic acid), generating energy in the form of two net molecules of ATP. Four molecules of ATP per glucose are actually produced, however, two are consumed as part of the preparatory phase. The initial phosphorylation of glucose is required to increase the reactivity (decrease its stability) in order for the molecule to be cleaved into two pyruvatemolecules by the enzyme aldolase. During the pay-off phase of glycolysis, four phosphate groups are transferred to ADP by substrate-level phosphorylation to make four ATP, and two NADH are produced when the pyruvate are oxidized. The overall reaction can be expressed this way:
- Glucose + 2 NAD+ + 2 Pi + 2 ADP → 2 pyruvate + 2 NADH + 2 ATP + 2 H+ + 2 H2O + heat
Starting with glucose, 1 ATP is used to donate a phosphate to glucose to produce glucose 6-phosphate. Glycogen can be converted into glucose 6-phosphate as well with the help of glycogen phosphorylase. During energy metabolism, glucose 6-phosphate becomes fructose 6-phosphate. An additional ATP is used to phosphorylate fructose 6-phosphate into fructose 1,6-disphosphate by the help of phosphofructokinase. Fructose 1,6-diphosphate then splits into two phosphorylated molecules with three carbon chains which later degrades into pyruvate.
Glycolysis can be literally translated as “sugar splitting”.
Oxidative decarboxylation of pyruvate
Pyruvate is oxidized to acetyl-CoA and CO2 by the pyruvate dehydrogenase complex (PDC). The PDC contains multiple copies of three enzymes and is located in the mitochondria of eukaryotic cells and in the cytosol of prokaryotes. In the conversion of pyruvate to acetyl-CoA, one molecule of NADH and one molecule of CO2 is formed.
Citric acid cycle
This is also called the Krebs cycle or the tricarboxylic acid cycle. When oxygen is present, acetyl-CoA is produced from the pyruvate molecules created from glycolysis. Once acetyl-CoA is formed, aerobic or anaerobic respiration can occur. When oxygen is present, the mitochondria will undergo aerobic respiration which leads to the Krebs cycle. However, if oxygen is not present, fermentation of the pyruvate molecule will occur. In the presence of oxygen, when acetyl-CoA is produced, the molecule then enters the citric acid cycle (Krebs cycle) inside the mitochondrial matrix, and is oxidized to CO2 while at the same time reducing NAD to NADH. NADH can be used by the electron transport chain to create further ATP as part of oxidative phosphorylation. To fully oxidize the equivalent of one glucose molecule, two acetyl-CoA must be metabolized by the Krebs cycle. Two waste products, H2O and CO2, are created during this cycle.
The citric acid cycle is an 8-step process involving 18 different enzymes and co-enzymes. During the cycle, acetyl-CoA (2 carbons) + oxaloacetate (4 carbons) yields citrate (6 carbons), which is rearranged to a more reactive form called isocitrate (6 carbons). Isocitrate is modified to become α-ketoglutarate (5 carbons), succinyl-CoA, succinate, fumarate, malate, and, finally, oxaloacetate.
The net gain of high-energy compounds from one cycle is 3 NADH, 1 FADH2, and 1 GTP; the GTP may subsequently be used to produce ATP. Thus, the total yield from 1 glucose molecule (2 pyruvate molecules) is 6 NADH, 2 FADH2, and 2 ATP.
Oxidative phosphorylation
In eukaryotes, oxidative phosphorylation occurs in the mitochondrial cristae. It comprises the electron transport chain that establishes a proton gradient (chemiosmotic potential) across the boundary of inner membrane by oxidizing the NADH produced from the Krebs cycle. ATP is synthesized by the ATP synthase enzyme when the chemiosmotic gradient is used to drive the phosphorylation of ADP. The electrons are finally transferred to exogenous oxygen and, with the addition of two protons, water is formed.



